ocrevus start up form
Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300. It is a one-time registration completed by the practice.
/multiple-sclerosis-ms-disability-5200983-Final-V2-e60aa676fe194a32a2e98eb49f859a0c.jpg)
Multiple Sclerosis Ms Disability Benefits Criteria Applying
Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services it.
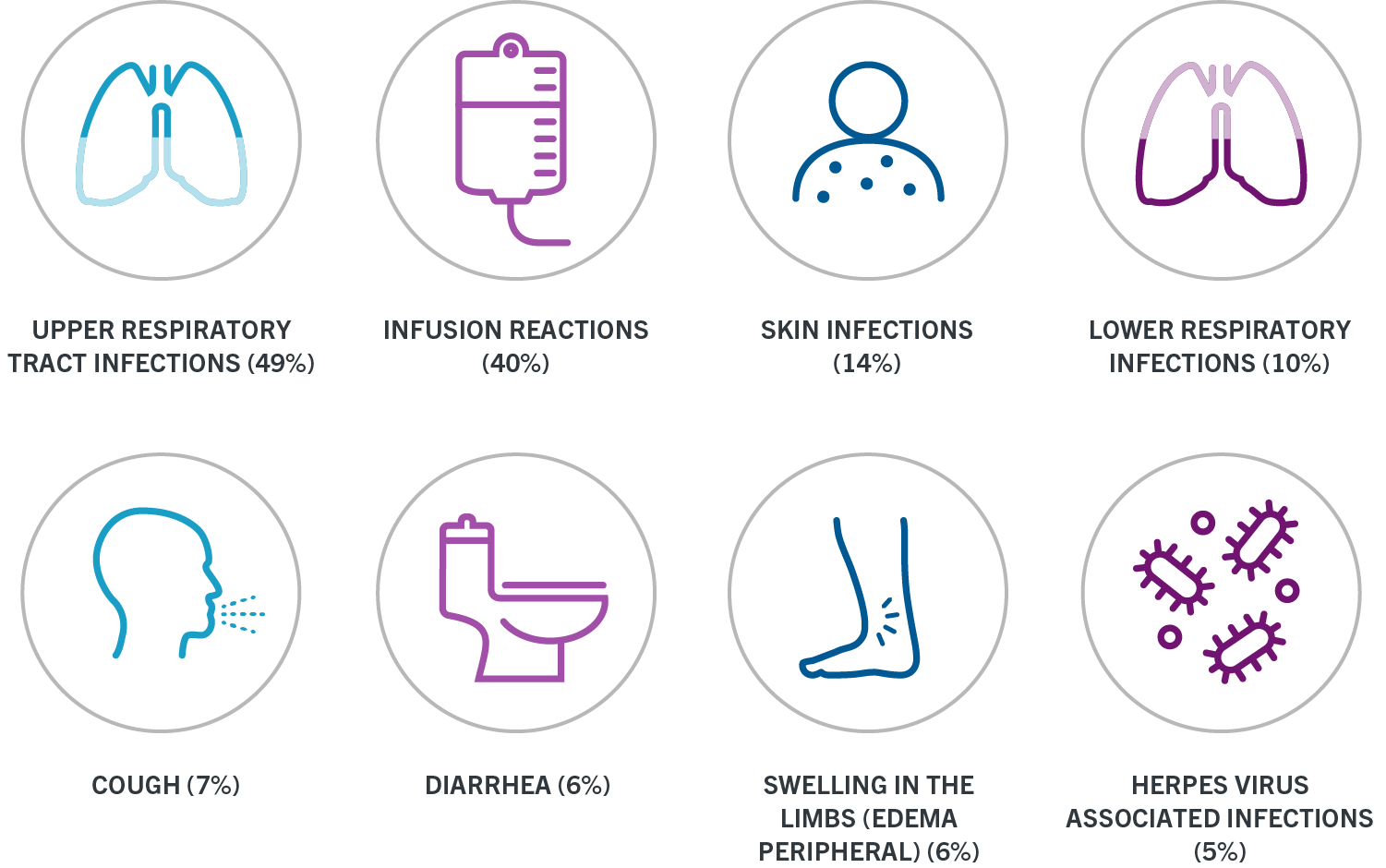
. Inform patients that infusion reactions can occur up to 24. Access Solutions is committed to helping your patients access the Genentech medicines they need providing assistance to your patients after OCREVUS is prescribed. Content updated daily for ocrevus start form.
Swelling of the throat. Each vial contains 300 mg10 mL of OCREVUS for intravenous infusion. 300 mg250 ml NS IV on day 1 and 15 then start maintenance dosing every 6 months.
Access the OCREVUS Start Form and learn more about the assistance Genentech offers for your OCREVUS ocrelizumab patients. OCREVUS ocrelizumab. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and.
These infusion reactions can happen for up to 24 hours after your infusion. And sign and date the form or it could delay our ability to help you. Ad Get patients started with AUBAGIO.
OCREVUS is a prescription medicine used to treat. Prior to the start of the intravenousinfusion the content of the infusion bag shouldbe at room temperature. See Website For Safety Boxed Warning PI.
Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. OCREVUS is supplied as a preservative-free sterile solution in a single-dose vial. Use the prepared infusion solution.
Visit The Access Solutions Site. View full prescribing information and Boxed Warning. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with.
Ocrevus Order Form Prescriber Signature Date Please Print Name Form 350 N. OCREVUS is administered by. It is important that.
Ad Discover The Safety Efficacy Of TYSABRI. To a final concentration of 12mgmL. Ad Discover The Safety Efficacy Of TYSABRI.
Every 6 months infuse 600mg in 500mL of 09 NS. Ad Looking for ocrevus start form. Ad Get Reimbursement Coding Info For Your Patients Here.
Visit The Access Solutions Site. This form is used to initiate the EFT registration process when the practice chooses not to use check reimbursements. View full prescribing information and Boxed Warning.
Instructions for Patients Please write legibly and complete all required fields on the OCREVUS Start Form to prevent delays. Ad Get Reimbursement Coding Info For Your Patients Here. See Website For Safety Boxed Warning PI.
These infusion reactions can happen for up to 24 hours after your infusion. Ad Get patients started with AUBAGIO.
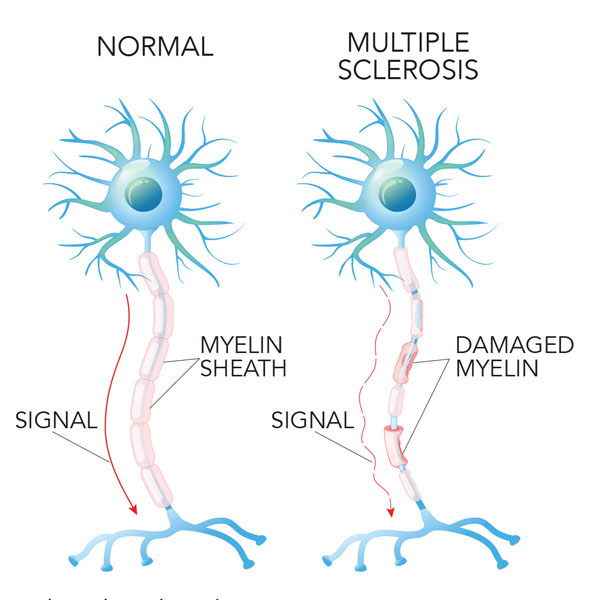
Multiple Sclerosis Symptoms And Causes Neurology Beaumont Health

Cognitive Impairment In Multiple Sclerosis Clinical Management Mri And Therapeutic Avenues The Lancet Neurology

Sign Up For Patient Support And Resources Ocrevus Ocrelizumab

Ocrevus Ocrelizumab Ms Infusion Experience

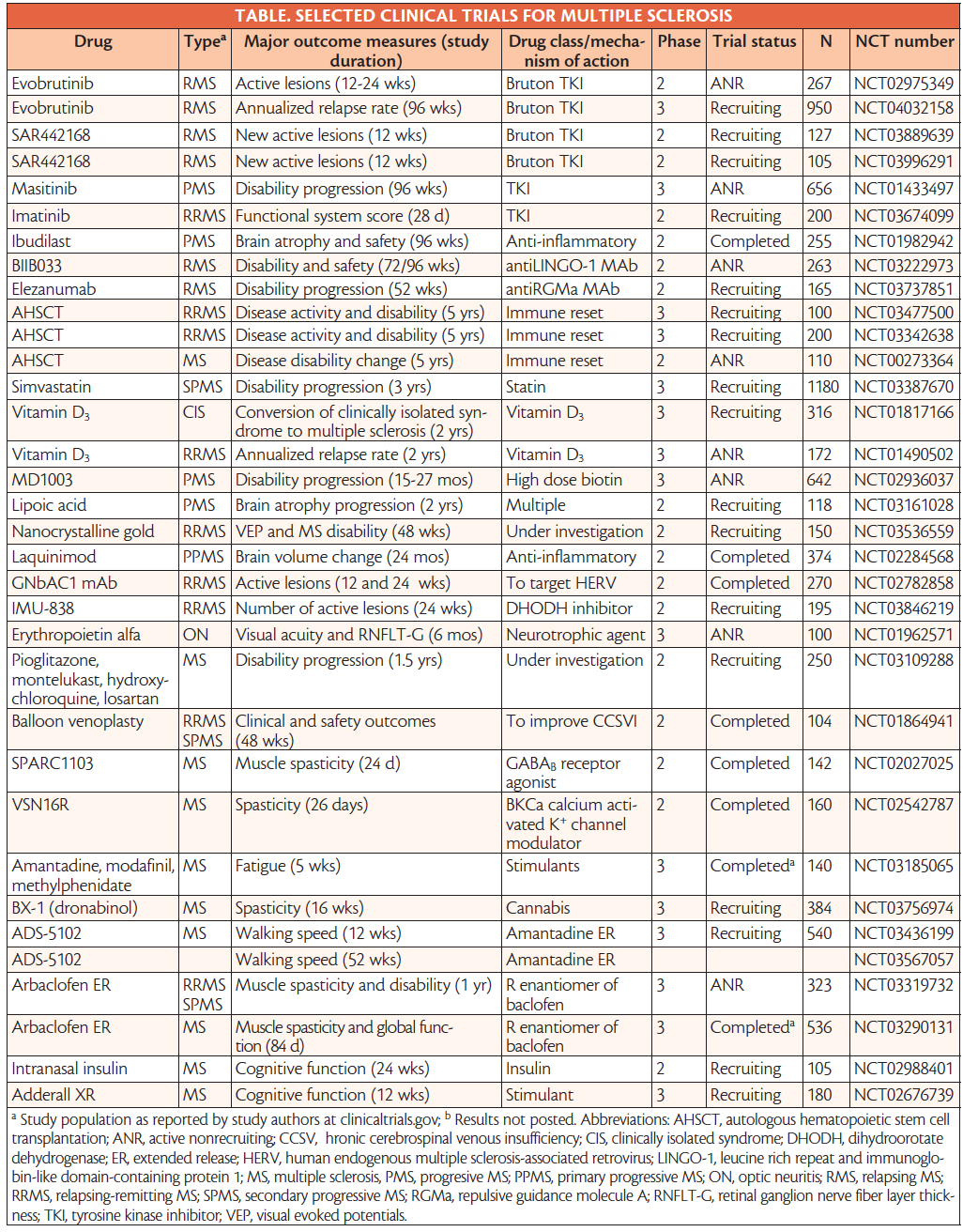
Drugs In Development For Multiple Sclerosis Practical Neurology
Ocrevus Connects Patient Support Program

Chronically Ill Traumatically Billed The 123 000 Medicine For Ms Kaiser Health News

Ocrevus Connects Patient Support Program
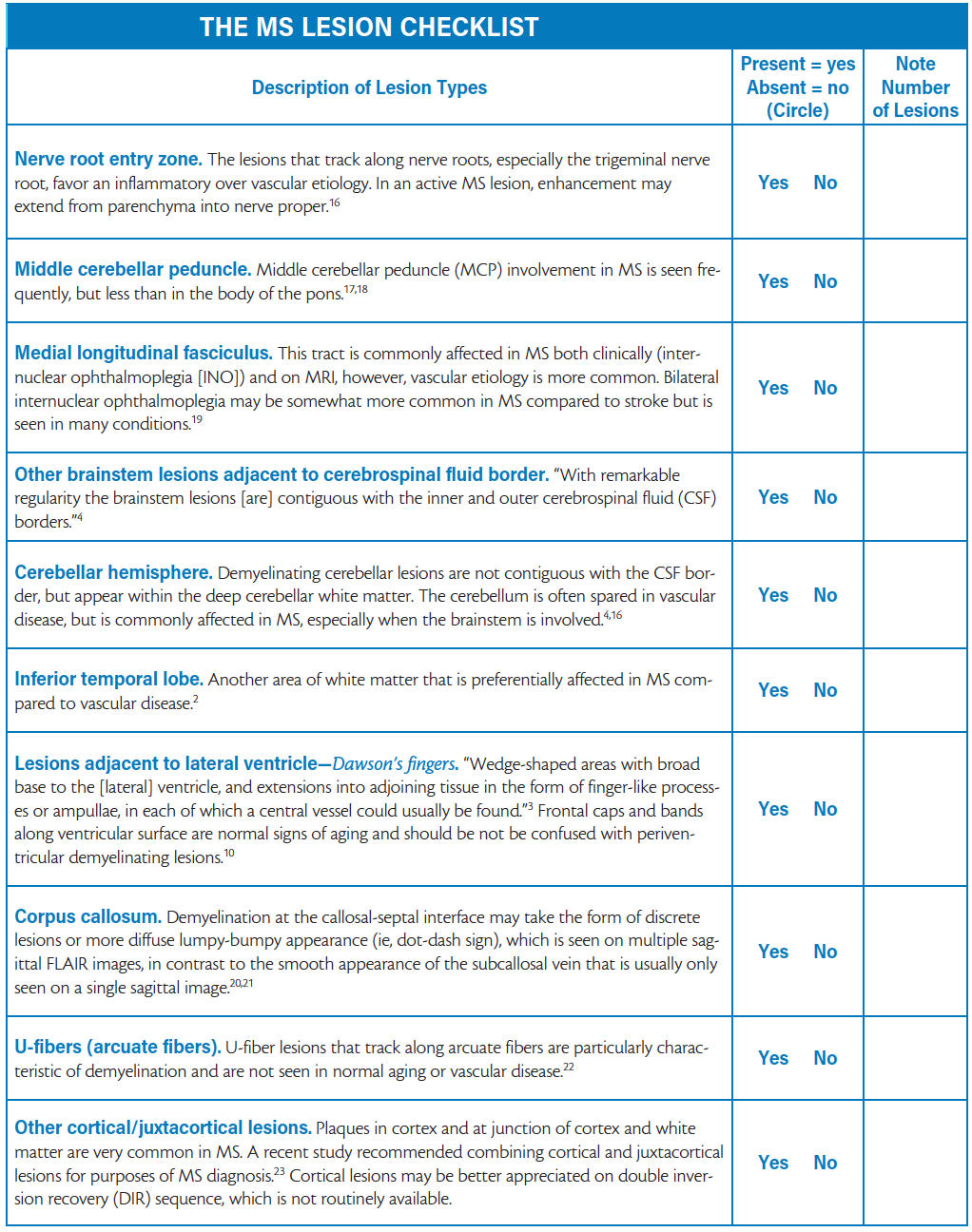
The Multiple Sclerosis Lesion Checklist Practical Neurology
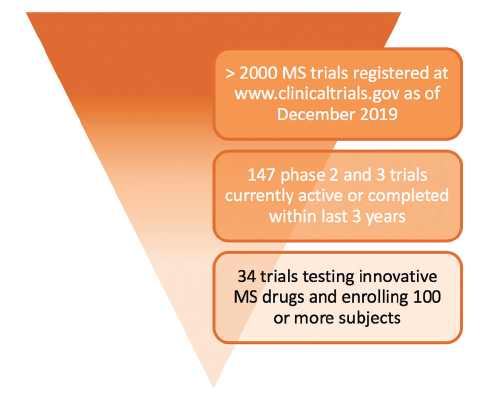
Drugs In Development For Multiple Sclerosis Practical Neurology
![]()
Ocrevus Connects Patient Support Program
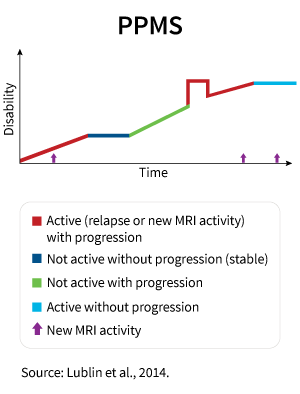
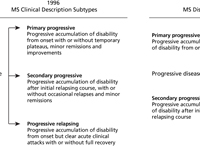
Primary Progressive Ms Ppms National Multiple Sclerosis Society

Ocrevus Ocrelizumab Ms Infusion Experience